Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. / Chemistry Lab: Types Of Chemical Reactions | Chemistry ... / The combination of 2 or more simple substances to form a more complex substance element +element = compound ex:
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. / Chemistry Lab: Types Of Chemical Reactions | Chemistry ... / The combination of 2 or more simple substances to form a more complex substance element +element = compound ex:. It can be more easily explained when you look at the mix of four ions which produces an insoluble product. Learn about the different types of chemical reactions and get examples of the reaction types. In this type of reaction, two or more reactants combine to synthesize a product. Doc brown's chemistry revision notes: Terms in this set (5).
Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: Classify the following word equations as representing either single or double displacement. Single displacement reaction a figure 2 in a single displacement reaction, one element, a, displaces element b in a compound, bc. Define all five reaction types. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex:

Redox reactions therefore include combustion reactions, single displacement reactions, and most.
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound. Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: Synthesis reactions are reactions that occur when two different atoms or molecules interact to form a synthesis reactions are one of the major classes of chemical reactions, which include single displacement, double this general type of reaction is perhaps the most common in chemistry. Six types of decomposition reactions. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. Define all five reaction types. There are six types of chemical reactions: In this straightforward worksheet, students are given a written chemical reaction, and asked to identify whether it's an example of synthesis, decomposition, single displacement, or double displacement. Neutralization reaction:neutralization reaction is a type of chemical reaction in which an acid and a base react to form salt and water. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: 149 999 просмотров 149 тыс. This is how the chemical equation of this reaction looks like. This chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions, combustion.
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. It can be more easily explained when you look at the mix of four ions which produces an insoluble product. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. The four major types of reactions.
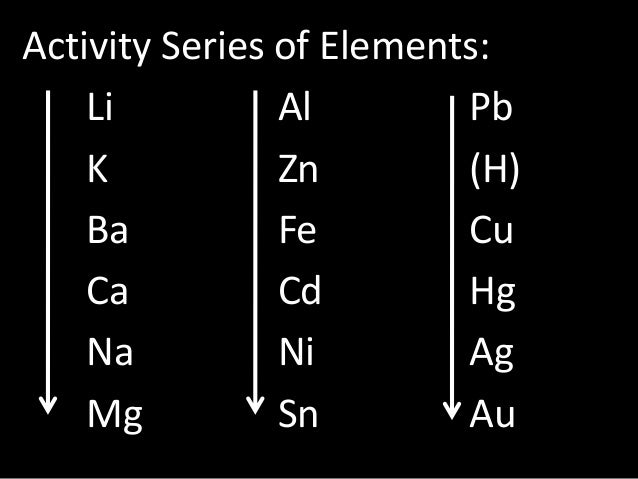
Decomposition reactions involve a single reactant breaking down to form two or more products.
Decomposition reactions involve a single reactant breaking down to form two or more products. Single replacement (or substitution or displacement) reactions. There are six types of chemical reactions: A decomposition reaction is called the. A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound. Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: Another type of chemical reactions is double displacement, in which the cations of the two. Transcribed image text from this question. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. Classify the following word equations as representing either single or double displacement. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. Define all five reaction types.
The four major types of reactions. This is a double replacement reaction. Physical and chemical changes during chemical reactions. What are their different types. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions.
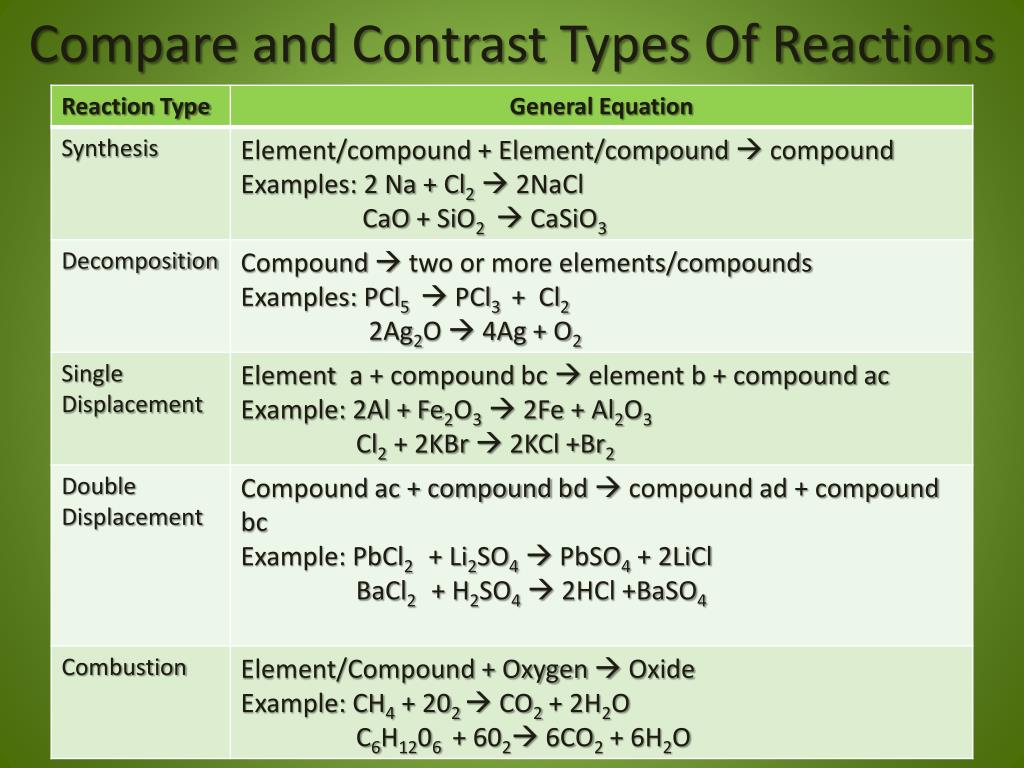
Single displacement reaction a figure 2 in a single displacement reaction, one element, a, displaces element b in a compound, bc.
On heating above 340°c, it decomposes to form two gases such as ammonia and. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. 149 999 просмотров 149 тыс. What are their different types. Classify the following word equations as representing either single or double displacement. A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound. This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. This gas then reacts vigorously with hot magnesium metal. The four major types of reactions. The thermal decomposition of ammonium chloride is a reversible chemical change. Single displacement reaction a figure 2 in a single displacement reaction, one element, a, displaces element b in a compound, bc. A decomposition reaction is called the. Another type of chemical reactions is double displacement, in which the cations of the two.
Komentar
Posting Komentar